1. Mold certification - Validation procedure for injection molds
The first step in validating an injection mold according to injection mold validation flow chart is mold certification.
Purpose:
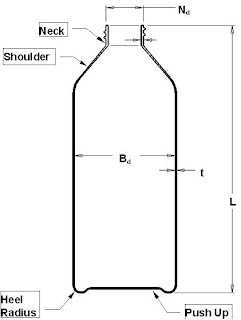
The purpose of the mold certification is to ensure the mold is built according to specification before the mold validation procedures begin, e.g., critical dimensions on the first mold set are cut "Steel Safe", core/cavity stack up dimensions are correct, cavity to cavity spacing is correct, cooling circuit layout, etc. The tool builder is required to provide verification of all critical metal tolerances on core and cavity ‘fits’ to be +/- 0,005 mm. The critical part dimension metal tolerances must be less than 10% of the plastics part dimension tolerances. In many cases, it will be necessary to go tighter than 10%. The mold certification should be performed at the tool builder while the mold is being fabricated to expedite any needed adjustments to the mold. Mold certification needs to be agreed upon when the mold order is placed. A copy of the mold certification should be included with the final mold validation report.
Variation observed during the process stability test, balance of fill analysis, and commissioning (multi-cavity analysis) are often a result of variation observed in the mold metal dimensions. Some molds, such as a four-face stack mold with a part thickness of approximately 0,80 mm are extremely sensitive to such variation, and may not pass the process stability test as a result. It is important for this step to be completed first so any correlation between variation observed during the remaining tests can be determined without pulling the mold from the press and disassembling it. Ensuring consistent cavity to cavity mold metal dimensions before running the mold will significantly decrease the time required to complete the validation procedure.
Data obtained from the mold certification for critical dimensions can be plotted on a control chart along with the critical part dimension obtained during the to determine if the two correlate to each other. A correlation between part dimension and steel dimension variation between cavities can be seen in figure:Correlation between steel and part dimensions
 Table:
Table: Steel certification summary
 summarize the metal certification data for this stack-up in figure:
summarize the metal certification data for this stack-up in figure: Critical core/cavity stack-up metal dimensions  The mold builder did not achieve their pre-determined mold metal tolerances. In some instances this may be acceptable. As shown for the outside diameter (dimension No. 5). A more constructive method to view the metal certification data is to control chart (X-MR) the data. The mold builder should know their measurement variation when performing metal certification. If not, they should perform a Gage repeatability & reproducibility (R&R) test. Ideally, the mold builder will machine the dimensions in control and be well within the agreed upon specifications.
The mold builder did not achieve their pre-determined mold metal tolerances. In some instances this may be acceptable. As shown for the outside diameter (dimension No. 5). A more constructive method to view the metal certification data is to control chart (X-MR) the data. The mold builder should know their measurement variation when performing metal certification. If not, they should perform a Gage repeatability & reproducibility (R&R) test. Ideally, the mold builder will machine the dimensions in control and be well within the agreed upon specifications.
The further steps are required in validating a injection mold according to injection mold validation flow chart:
2. Dry cycle mold
3. Process stability test
4. Gage repeatability & reproducibility (R&R) test
5. Mold viscosity test
6. Balance of fill analysis
7. Gate freeze test
8. Commissioning (multi-cavity analysis)
9. Design of experiments
10. Qualification (process capability study)
11. Mold metal Adjustments - centering process
12. Verification (30-day run)
Purpose:
The purpose of the mold certification is to ensure the mold is built according to specification before the mold validation procedures begin, e.g., critical dimensions on the first mold set are cut "Steel Safe", core/cavity stack up dimensions are correct, cavity to cavity spacing is correct, cooling circuit layout, etc. The tool builder is required to provide verification of all critical metal tolerances on core and cavity ‘fits’ to be +/- 0,005 mm. The critical part dimension metal tolerances must be less than 10% of the plastics part dimension tolerances. In many cases, it will be necessary to go tighter than 10%. The mold certification should be performed at the tool builder while the mold is being fabricated to expedite any needed adjustments to the mold. Mold certification needs to be agreed upon when the mold order is placed. A copy of the mold certification should be included with the final mold validation report.
Variation observed during the process stability test, balance of fill analysis, and commissioning (multi-cavity analysis) are often a result of variation observed in the mold metal dimensions. Some molds, such as a four-face stack mold with a part thickness of approximately 0,80 mm are extremely sensitive to such variation, and may not pass the process stability test as a result. It is important for this step to be completed first so any correlation between variation observed during the remaining tests can be determined without pulling the mold from the press and disassembling it. Ensuring consistent cavity to cavity mold metal dimensions before running the mold will significantly decrease the time required to complete the validation procedure.
Data obtained from the mold certification for critical dimensions can be plotted on a control chart along with the critical part dimension obtained during the to determine if the two correlate to each other. A correlation between part dimension and steel dimension variation between cavities can be seen in figure:
 Table:
Table:  summarize the metal certification data for this stack-up in figure:
summarize the metal certification data for this stack-up in figure:  The mold builder did not achieve their pre-determined mold metal tolerances. In some instances this may be acceptable. As shown for the outside diameter (dimension No. 5). A more constructive method to view the metal certification data is to control chart (X-MR) the data. The mold builder should know their measurement variation when performing metal certification. If not, they should perform a Gage repeatability & reproducibility (R&R) test. Ideally, the mold builder will machine the dimensions in control and be well within the agreed upon specifications.
The mold builder did not achieve their pre-determined mold metal tolerances. In some instances this may be acceptable. As shown for the outside diameter (dimension No. 5). A more constructive method to view the metal certification data is to control chart (X-MR) the data. The mold builder should know their measurement variation when performing metal certification. If not, they should perform a Gage repeatability & reproducibility (R&R) test. Ideally, the mold builder will machine the dimensions in control and be well within the agreed upon specifications.The further steps are required in validating a injection mold according to injection mold validation flow chart:
2. Dry cycle mold
3. Process stability test
4. Gage repeatability & reproducibility (R&R) test
5. Mold viscosity test
6. Balance of fill analysis
7. Gate freeze test
8. Commissioning (multi-cavity analysis)
9. Design of experiments
10. Qualification (process capability study)
11. Mold metal Adjustments - centering process
12. Verification (30-day run)


Comments